From volume 92, spring 2010
By Kisa Elmer and Cora Shea
For those of you who have ever stopped to peer down at a single surface hoar crystal, the beauty of self assembly has already been revealed to you. The ability of water vapour to self-assemble into tiny ice sculptures still has scientists and curious observers searching for answers, and winter recreationists dodging avalanche trigger zones.
As avalanche safety workers reflect on the tragedies of past winters, surface hoar formation and the persistent weak layer that follows its burial appear to be poorly understood. So a research group like ASARC asks itself, what can we contribute to industries and recreationists? Two contributions are a better understanding of avalanche triggering mechanics and improved methods of identifying risk. One area of ongoing research is predicting and mapping surface hoar formation.
Surface hoar formation has been a difficult, yet essential research topic. Difficult because the natural processes behind crystal formation—such as wind and humidity—are near impossible to model at the scales relevant to surface hoar growth. Essential because, once buried, the surface hoar layer creates an ideal failure plane.
In the summer of 2009, the Applied Snow and Avalanche Research group at the University of Calgary hired a research assistant to help with the grunt work of research. That assistant was me, Kisa Elmer, a civil engineering student from the University of Saskatchewan. During the first month of my summer research position with ASARC, I worked with Cora Shea analyzing wide angle photos of the sky above surface hoar samples at the Fidelity study site in Glacier National Park. The objective was to understand the relationship between crystal size and sky view, a term defined as “the amount of open sky available for snow surface longwave radiation losses at night.” (Shea and Jamieson, 2009). This was my introduction to the curious subject of surface hoar formation. The next few weeks were full of lunch time discussions on the effects of temperature, wind, radiation and humidity on crystal growth.
After helping analyze the photos and comparing crystal sizes to sample locations, a new task presented itself. It had not been done before and neither Cora nor I knew if it would be successful. We were going to grow our own surface hoar in the cold lab—an unlikely place for surface hoar to grow. One reason is that the cold lab is closed off, which means that air flow and radiation losses are limited. A second reason is that there was no snow available for the crystals to grow on, so a proper surface needed to be found.

Figure 1: Using basic equipment, the researchers worked to replicate a small sample of what nature does in incredible variety—make surface hoar.
The Experiment
On July 14, jumping head first into the experiment, we decided to do the intuitive thing; boil water, move it into the cold lab, trap it with a plastic container and simply let the natural phenomenon of self assembly take the reins. You can see the simple apparatus consisting of a pot of boiling water, a steel paint strainer, and two plastic paint strippers in Figure 1, all to be covered by a plastic bin. The apparatus was not sophisticated; there was no delicate thermocouple wires dedicated to providing temperature readings or expensive gadgets measuring relative humidity, yet, it was successful. After leaving it overnight in a dark cold lab, devoid of sky view and significant air flow, tiny ice sculptures appeared on the underside of the steel paint strainer.
At different temperatures there would be, inevitably, different formations occurring. Plates formed at an ambient cold lab temperature of -7 ºC (Figure 2), while needles and feathery structures formed at -12 ºC and -13 ºC (Figure 3). A transition zone producing stretched plate structures was also observed from -9 ºC to -11 ºC. To give you an idea of size, the plates measure on the scale of a few millimetres while the needles grew to a maximum of one centimetre.

Figure 2
Here’s how we thought it would work, in theory. The air trapped under the bin would soon become saturated and condense around the equipment. As the steel paint strainer cooled with its surroundings, the condensation began to freeze around its edges, creating a good base for surface hoar growth. However, this is not exactly what happened. There was an inconsistency in the temperature. Outside the bin was cold (sometimes down to -13ºC). Inside the bin the air was kept warm from the hot pot of boiling water. In the same way our body reacts to cold, the extremities of the experiment cooled first, and because the edges of the bin were the first to reach freezing temperatures, vapour molecules began moving away from our nicely laid out landing pad and towards the plastic bin. The unruly vapour collected itself onto the unintentional surface, forming a less interesting layer of frost.
While humidity is an important factor in the formation of surface hoar it is not the only cause of crystal growth. Wind and sky view are two other contributors to their growth. If humidity were the only factor, we would have seen a more uniformly spread collection of crystals. Instead, the crystal growth was concentrated near the openings between the paint strippers and the edge of the pot, growing towards the vapour source. By positioning the paint strippers in a way that constricted steam flow through the strainer (Figure 1), an environment offering saturated air flow was created.
Conclusion
The imitation hoar grew under different circumstances and through a different process than natural surface hoar crystals. Simply put, natural surface hoar is formed when the snow surface cools enough at night to attract nearby water vapour molecules from the air. The imitation surface hoar grew because of highly saturated air flow. Our questions about the relationship between sky view and crystal growth still remain, as new questions were generated on the underlying physical processes governing the phenomenon of self-assembly. Perhaps this small experiment created more questions than it answered.
Now, after wrapping up the project and heading back to university, I still think about how to improve the experiment for future trials. Maybe I can try different surface materials such as natural snow, a different set up, or a different procedure. What factors can be controlled and manipulated other than temperature and what will be the result? When starting the experiment I wasn’t sure whether any relevant results would be possible. Ice and frost were a given, but surface hoar? I wasn’t sure. After observing the outcome, I wonder if this is the beginnings of a new way to study surface hoar formation and what it might mean to practitioners in the future.
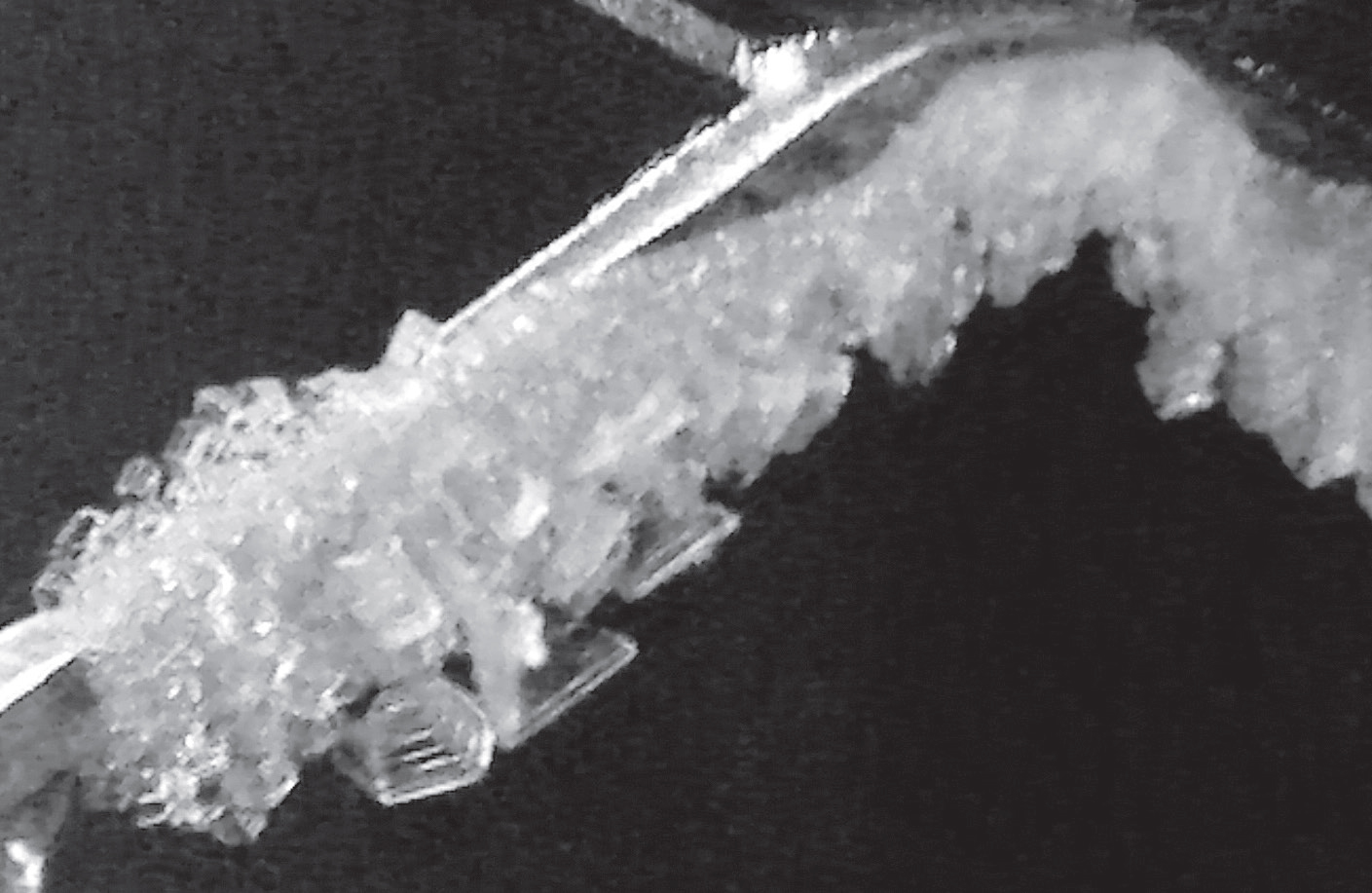
Figure 3
Acknowledgements:
Thanks to everyone involved, including all members of ASARC. Specifically, thanks to Cora Shea and Bruce Jamieson for all of their advice. Thank you to the Civil Engineering Department staff at Schulich School of Engineering who provided all the necessary equipment. I would not have been able to do this without the help of Terry Quin.
Reference:
Shea, C. and B. Jamieson. Predicting surface hoar spatial variability in sparse forests using shading in satellite imagery, International Snow Science Workshop, Swiss Federal Institute for Forest, Snow and Landscape Research WSL., Davos, Switzerland, p.102-106 (2009)






